| Projects | |
| Non-endosomal functions of ESCRT-III proteins | |
| The
endosomal sorting complex required for transport (ESCRT)-III proteins
are filamentous membrane remodeling factors that are part of an ancient
system whose main functions appears to be to bring membranes into close
contact. The basic components of the ESCRT-system, an ESCRT-III-like
protein and a protein homologous to the AAA-ATPase Vps4 were already
present in the "Last Common Eukaryotic Ancestor" and are still found in
all eukaryotic organisms to date. According to biochemical experiments, the ESCRT proteins can be grouped into several protein complexes, called ESCRT-0, -I, -II and -III. It is thought that these protein complexes function consecutively in the recruitment and packaging of endocytic cargo proteins - bound for degradation in the lysosome or yeast vacuole - into vesicles that bud into the interior of the endosome. In the process of intraluminal vesicle (ILV) formation, ESCRT-III in conjunction with Vps4 is involved in the abscission step, i.e. the release of the vesicle from the membrane into the interior of the endosome. In yeast, the classical ESCRT-III protein family consists of six small hydrophilic, mostly α helical proteins. In addition, there are two larger proteins containing an ESCRT-III domain. There are counterparts to these eight proteins in human cells that are called CHMPs (CHarged Multivesicular body Protein) and IST1. Gene duplications in some of the groups led to an expansion of the gene family. The protein complex biochemically defined as "ESCRT-III" consists of four subunits, Snf7, Vps2, Vps20 and Vps24. The exact composition of the complex is unclear. The main component of ESCRT-III seems to be Snf7, which forms a circular filament surrounding the neck of the budding ILV. The propensity to form filaments seems to be a general property of ESCRT-III proteins. So far, homo- or hetero-oligomeric filaments were described for CHMP1B, CHMP2A, CHMP2B, Vps24 and CHMP3, Snf7 and CHMP4A, CHMP4B and for IST1. In the past few years, a plethora of new functions of ESCRT-III proteins has been discovered. In addition to their classical role in MVB sorting, ESCRT-III is involved in HIV-budding, cytokinesis, plasma membrane repair, resealing of the nuclear envelope after mitosis, repair of the nuclear envelope, autophagy, RNA virus replication, transport across the nuclear membrane and neuron pruning. 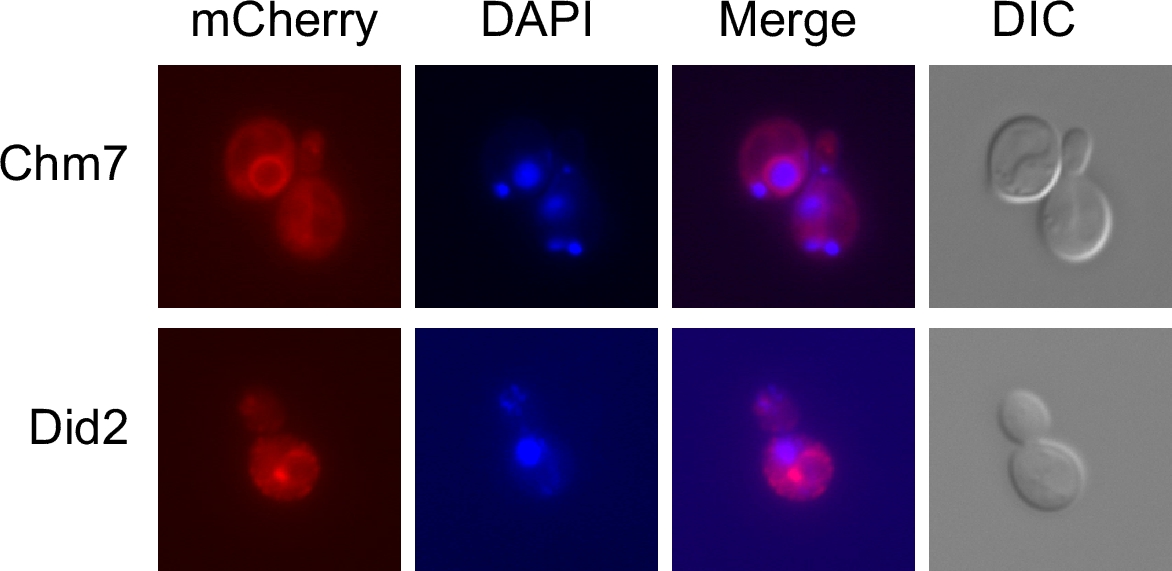 We now obtained evidence for the existence of an alternative ESCRT-III complex in yeast functioning at the endoplasmic reticulum. This complex contains the non-canonical ESCRT-III protein Chm7 as a subunit. Our goal is to unravel the function of the Chm7 complex and to study other new aspects of ESCRT-III function. |
|
|
Development of cellulolytic yeast strains for the production of second generation bioethanol
|
|
The use of starchy raw materials such as corn and grain for bioethanol production has faced a lot of criticism because of its alleged competition with food production. To escape this "food-or-fuel" debate, the conversion of lignocellulose-containing wastes and residues to bioethanol represents a promising alternative to the use of starchy raw materials. The conversion of lignocellulose to ethanol, however, is challenging and not yet competitive with first generation bioethanol.  |
|
To overcome the specific problems associated with the production of cellulosic ethanol and to make it more cost effective, we want to establish a simple, continuous process. Prerequisite for such a process is a production organism which can bring about the decomposition of lignocellulose and the conversion to ethanol in a single step. Our goal is therefore to develop a cellulolytic yeast strain based on the bacterial cellulosome that is able to efficiently hydrolyze cellulose without the addition of external enzymes. The digested biomass also represents a cost-effective growth substrate for cultivating microorganisms for biotechnological processes. In principle, the nutrients released from the biomass could be converted into any, industrially useful compound. Robust production organisms need to be developed for this purpose. Using genetic engineering, the desired metabolic pathways can be transferred into these organisms. |